Reading a Distillation Curve for Boiling Point
Partial Distillation of Ideal Mixtures
- Page ID
- 3869
This page explains how the fractional distillation (both in the lab and industrially) of an platonic mixture of liquids relates to their stage diagram.
Using the phase diagram
On the last page, nosotros looked at how the stage diagram for an ideal mixture of two liquids was congenital up. I want to commencement by looking again at material from the last role of that folio. The side by side diagram is new - a modified version of diagrams from the previous folio.
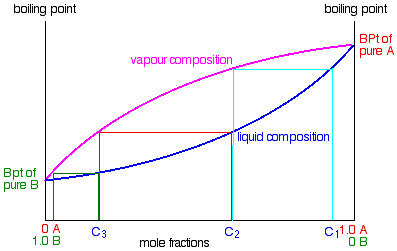
- If you boil a liquid mixture C1, you will go a vapor with limerick C2, which you tin can condense to give a liquid of that same limerick (the pale bluish lines).
- If you reboil that liquid C2, it will give a vapor with composition C3. Over again you lot can condense that to give a liquid of the same new composition (the cherry lines).
- Reboiling the liquid C3 will give a vapor still richer in the more volatile component B (the dark-green lines). You can run across that if you were to practise this in one case or twice more, you would be able to collect a liquid which was most pure B.
The secret of getting the more than volatile component from a mixture of liquids is obviously to do a succession of boiling-condensing-reboiling operations. Information technology is not quite so obvious how y'all get a sample of pure A out of this.
Fractional Distillation in the lab
A typical lab fractional distillation would await like this:
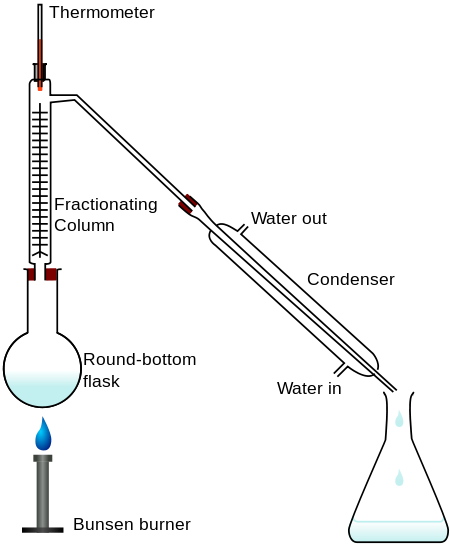
The fractionating cavalcade is packed with drinking glass beads (or something similar) to give the maximum possible surface expanse for vapor to condense on. Some fractionating columns accept spikes of glass sticking out from the sides which serve the same purpose. If you sketch this, brand sure that yous exercise not completely seal the apparatus. There has to be a vent in the arrangement otherwise the pressure build-up when you heat it volition blow the apparatus apart. In some cases, where you are collecting a liquid with a very low humid point, you may demand to surround the collecting flask with a chalice of cold water or ice. The mixture is heated at such a rate that the thermometer is at the temperature of the boiling indicate of the more volatile component. Notice that the thermometer seedling is placed exactly at the outlet from the fractionating column.
Relating what happens in the fractionating column to the phase diagram
Suppose you eddy a mixture with composition C1. The vapor over the pinnacle of the humid liquid will exist richer in the more than volatile component, and volition accept the composition Ctwo.
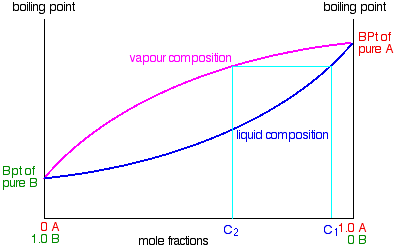
That vapor now starts to travel up the fractionating column. Eventually it will reach a peak in the cavalcade where the temperature is low enough that information technology volition condense to give a liquid. The composition of that liquid volition, of course, still exist C2. So what happens to that liquid at present? Information technology will offset to trickle down the column where it will meet new hot vapor rising. That volition crusade the already condensed vapor to reboil.

Some of the liquid of composition C2 will boil to give a vapor of composition C3. Allow's concentrate outset on that new vapor and think virtually the unvaporized part of the liquid later.
The vapor
This new vapor volition again move further up the fractionating column until it gets to a temperature where it can condense. And then the whole process repeats itself. Each time the vapor condenses to a liquid, this liquid will get-go to trickle dorsum down the cavalcade where information technology will be reboiled by up-coming hot vapor. Each fourth dimension this happens the new vapor will be richer in the more volatile component. The aim is to balance the temperature of the cavalcade so that by the fourth dimension vapor reaches the pinnacle later on huge numbers of condensing and reboiling operations, it consists only of the more than volatile component - in this case, B.
Whether or not this is possible depends on the divergence between the boiling points of the two liquids. The closer they are together, the longer the column has to be.
The liquid
So what near the liquid left behind at each reboiling? Obviously, if the vapor is richer in the more volatile component, the liquid left backside must exist getting richer in the other ane. As the condensed liquid trickles down the column constantly being reboiled by upwardly-coming vapor, each reboiling makes it richer and richer in the less volatile component - in this example, A. By the time the liquid drips back into the flask, information technology volition exist very rich in A indeed. And then, over time, as B passes out of the top of the column into the condenser, the liquid in the flask will get richer in A. If y'all are very, very careful over temperature control, eventually yous volition take separated the mixture into B in the collecting flask and A in the original flask. Finally, what is the indicate of the packing in the column?
To make the humid-condensing-reboiling process as effective as possible, it has to happen over and over again. By having a lot of area inside the cavalcade, yous aim to have the maximum possible contact betwixt the liquid trickling down and the hot vapor rising. If y'all didn't have the packing, the liquid would all exist on the sides of the condenser, while most of the vapor would be going upwardly the center and never come into contact with information technology.
Fractional distillation industrially
There is no departure whatsoever in the theory involved. All that is different is what the fractionating column looks similar. The diagram shows a simplified cantankerous-section through a small part of a typical column.

The column contains a number of trays that the liquid collects on as the vapor condenses. The up-coming hot vapor is forced through the liquid on the trays by passing through a number of bubble caps. This produces the maximum possible contact between the vapor and liquid. This all makes the boiling-condensing-reboiling process as efficient as possible. The overflow pipes are simply a controlled style of letting liquid trickle down the column.
If you have a mixture of lots of liquids to split up (such equally in petroleum fractionation), information technology is possible to tap off the liquids from some of the trays rather than simply collecting what comes out of the top of the column. That leads to simpler mixtures such equally gasoline, kerosene and and so on.
Example ane
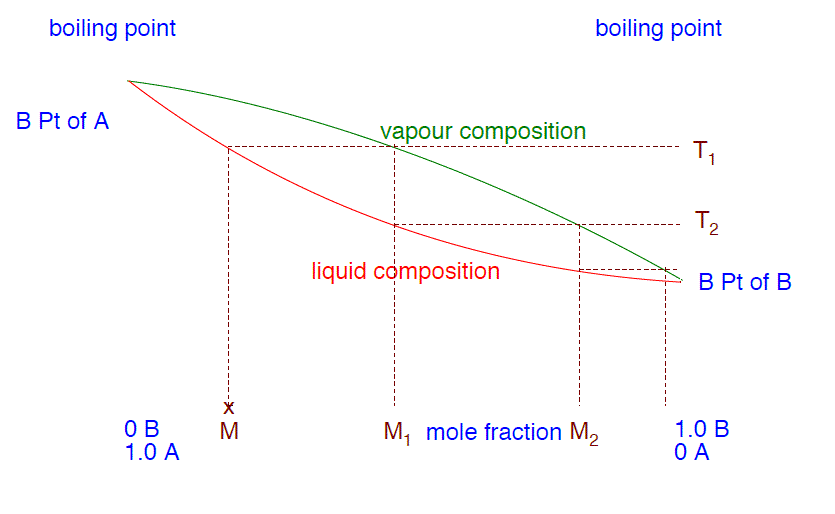
- Utilize the phase diagram beneath to explain how y'all tin obtain a pure sample of B from a mixture M by successively humid and condensing the liquid mixture.
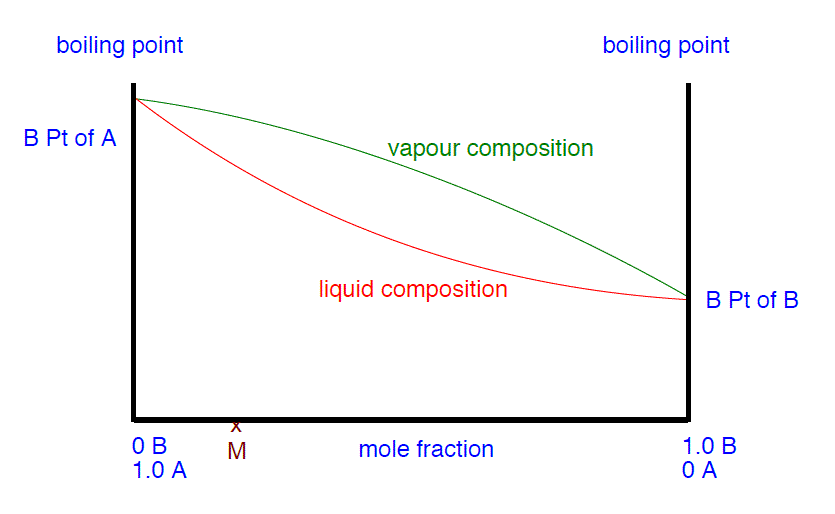
- Why is it important to carefully control how strongly the original mixture is heated during the separation?
- Explicate briefly how the separation occurs, making use of the phase diagram above if you think it helps.
Solution
- If you eddy the mixture M, it will boil at a temperature T1. The vapor above the liquid at this temperature will be richer in the more volatile substance B. If yous condense that vapor, it volition give a liquid of the composition M1. If you reboil that, it will boil at a temperature T2. The vapor over that liquid will have a composition M2, still richer in B. If you proceed doing that, reboiling and recondensing, then the vapor becomes richer and richer in B until it eventually becomes pure B. When you lot finally become to that point and condense the vapor, so you will have pure B liquid.
- You lot have to be sure that just the vapor of the more volatile of the two liquids passes into the condenser. That means that the thermometer has to read exactly the boiling point of the more volatile liquid. If information technology is beneath that, so nothing is going to pass out into the condenser. If it is above that, and so your distillate will still contain some of the less volatile component.
- B is the more than volatile liquid; A is the less volatile one. The vapor over the boiling liquid in the flask volition be richer in B than the original liquid is. That vapor will turn down the column until the temperature falls enough for information technology to condense to give a liquid richer in B than the one in the flask (equivalent to M1 in the diagram). This will start to trickle downwardly the column. Hot vapor coming up from the flask will reboil the condensed liquid, giving a vapor which will exist even richer in B (M2 on the diagram). This will condense to a liquid, trickle downwards the cavalcade and and so be reboiled. This continuous procedure will go on until the vapor is entirely B. The column is heated then that this is finally complete correct at the height of the column. Meanwhile, the liquids trickling down the column get richer and richer in A as the B is removed and carried upward the column. Eventually, the liquid in the flask will terminate up as pure A.
concepcionwoustravight1945.blogspot.com
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Physical_Equilibria/Fractional_Distillation_of_Ideal_Mixtures
0 Response to "Reading a Distillation Curve for Boiling Point"
Post a Comment